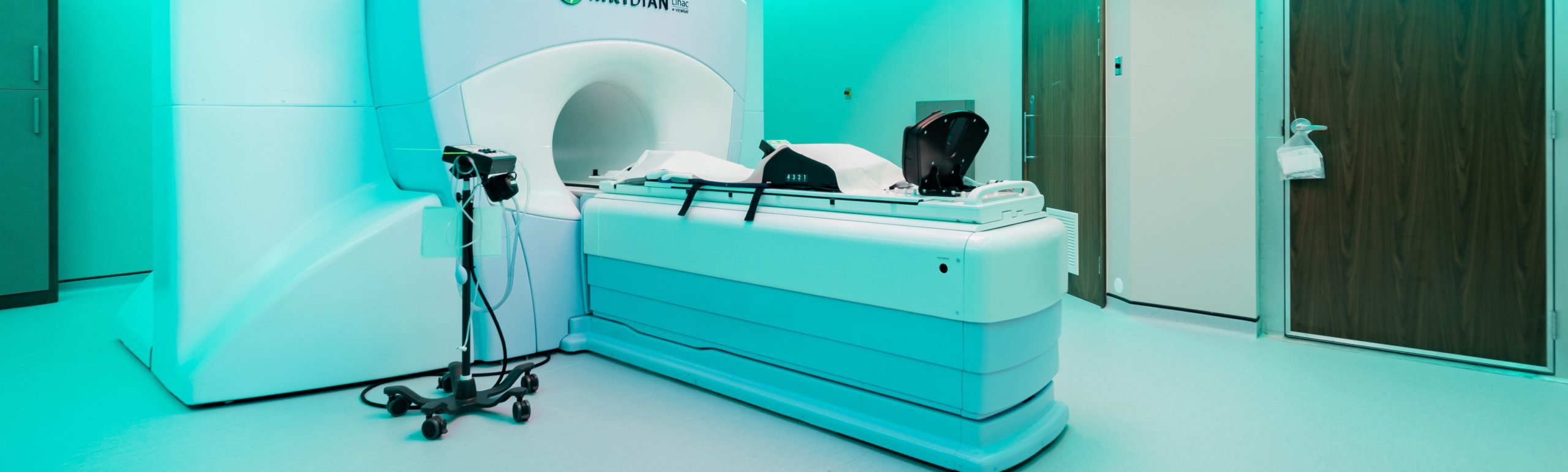
The patients will be treated with ‘stereotactic ablative radiotherapy’ (SABR) using the UK’s first MRIdian® machine at a private healthcare facility in Oxford. The MRIdian system uses magnetic resonance imaging (MRI) to provide live images of the tumour and surrounding tissue to enable the radiotherapy to be delivered with pinpoint precision. Uniquely, it also adjusts the radiation beams in real time to the tiniest change in the tumour’s position, such as movement from breathing. Such precision reduces the risk of radiation damaging healthy tissues around the tumour, which potentially means higher doses can be delivered over fewer treatments.
SABR is currently used to treat some types of cancer on the NHS – but not pancreatic cancer, because more evidence of its potential benefits over conventional radiotherapy for patients needs to be gathered for it to be commissioned.
 As a result, the UK independent healthcare provider GenesisCare is providing its state-of-the-art MRIdian facilities at its Oxford Centre at no cost to the NHS, as part of a Compassionate Access Programme. The data gathered will act as a pilot phase for clinical trials planned by the University of Oxford once COVID-19 related restrictions are lifted, to assess the effectiveness of this treatment. The programme also ensures that patients taking part whose surgery or chemotherapy has been postponed during the COVID-19 pandemic will have access to a viable treatment option.
As a result, the UK independent healthcare provider GenesisCare is providing its state-of-the-art MRIdian facilities at its Oxford Centre at no cost to the NHS, as part of a Compassionate Access Programme. The data gathered will act as a pilot phase for clinical trials planned by the University of Oxford once COVID-19 related restrictions are lifted, to assess the effectiveness of this treatment. The programme also ensures that patients taking part whose surgery or chemotherapy has been postponed during the COVID-19 pandemic will have access to a viable treatment option.
Dr James Good, Clinical Oncologist and Clinical Director of SABR at GenesisCare said: “The MRIdian machine is at the cutting-edge of what is possible in radiotherapy technology. The ability to visualise the tumour more accurately, to follow it while it’s being treated and to adapt the plan every day means we can deliver the best possible outcomes.”
Eligible NHS patients throughout the UK can be referred via their NHS consultant, and if accepted onto the programme, will have five radiotherapy sessions at GenesisCare Oxford.
Tim Maughan, Professor of Clinical Oncology at the University of Oxford said: “There are two ways that we’ll look at the outcomes of this project. The first is by asking referring clinicians to let us know whether the cancer is stable on the scans and how well people do in terms of side effects. We’ll also be asking patients to report side effects and other impacts of the treatment on their quality of life. We’re delighted that we’ve been able to enrol the first few patients already.”
The partners in this programme are GenesisCare Foundation (the charitable arm of GenesisCare); ViewRay, which produces the MRIdian machines; researchers and clinicians from the University of Oxford and PCRF.
Says Maggie Blanks, PCRF’s CEO: “We’re proud to partner this important research programme. We need more treatment options for pancreatic cancer patients, and if SABR is a treatment that offers better outcomes, then we need that evidence as soon as possible.”
Furthermore, so that the programme in Oxford can provide access to patients from across the UK, PCRF will cover the cost of essential travel and accommodation for the duration of the treatments, to ensure that no eligible patient is prevented from participating.
To find out more about the project, please take a look at this video: